I apologize for the state of the bibliography: there are some formatting errors that I will correct when I have the time.
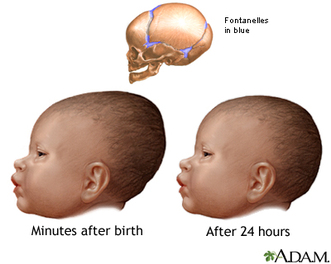 Figure 1. Illustration of changes in head shape that occur during birth (from A.D.A.M).
Figure 1. Illustration of changes in head shape that occur during birth (from A.D.A.M). This study compares available data from fetal and neonatal crania from the Late Pleistocene to the mechanics of fetal head molding during birth in recent humans. The small number of fetal and neonatal remains dating to the Late Pleistocene offer an opportunity to simultaneously explore issues of obstetrics, selection, and early brain growth. Most treatments of birth and obstetrics in Pleistocene humans have focused on pelvic anatomy (e.g., Rak and Arensburg 1987; Rosenberg 1998; Rosenberg and Trevathan 2002; Trinkhaus 1984). Studies of childhood growth and development after birth are limited mainly by the dearth of sub-adult skeletons, particularly those that pre-date Neandertals (see Anton 2002; Dean et al. 1986; Minugh-Purvis 1988, 2002; Nelson and Thompson 2002; Stringer et al. 1990; Tellier 1998; Trinkhaus and Tompkins 1990). Fetal and neonatal remains, from Neandertals and other Late Pleistocene humans, have been described but have not been the subject of detailed, hypothesis-based research.
Deformation (molding) of the fetal cranium is an important part of successful birth in recent humans. This study examines the hypothesis that thickness of fetal cranial bone would have been an impediment to successful birth in Neandertals and other Late Pleistocene humans. Investigating the possible role of fetal head molding in Pleistocene obstetrics may help shed light on both anatomical trends in human cranium (i.e., the emergence of "modern" cranial morphology) and demographic variables and population genetics that may underlay the spread of anatomical "modernity." Mortality and trauma during childbirth, acting on the mother and/or the fetus, would be an important selective force.
Two aspects of fetal head molding are emphasized: cranial vault thickness and head dimensions. Vault thickness affects the response of the cranium to pressure during birth. Head size and shape affect both the degree of molding that is required for the fetal head to pass through the birth canal and the distribution of forces on the fetal cranium.
Hypothesis: Thick fetal cranial bone in Late Pleistocene archaic humans would have caused difficulties during childbirth (relative to recent humans) by inhibiting head molding during delivery.
Assuming uniformity in the size of the birth canal between archaic and recent humans, this hypothesis has two test implications:
1) the increased thickness of Late Pleistocene fetal cranial vaults would have a significant effect on elasticity of the cranium
2) the dimensions of the fetal cranium are such that significant molding is required for delivery
In other words, fetal head molding must be shown to be both necessary (by the dimensions of the cranium) and significantly impeded (by the in-elasticity of the vault) in order to fail to reject the hypothesis. If either one of these test implications is rejected, then the hypothesis can be rejected.
Much research focused on questions of obstetrics in Pleistocene humans have emphasized the selective constraints between locomation and birth mechanics in the pelvis (Rak and Arensburg 1987; Rosenberg 1998; Rosenberg and Trevathan 2002; Ruff 1995; Trinkhaus 1984). Based on pelvis remains, most researchers conclude that birth in Pleistocene humans was much like birth in recent humans (Rosenberg 1998; Rosenberg and Trevathan 2002). Subsequent to the description of the Kebara 2 pelvis (Rak and Arensburg 1987), most ideas about an unusually long gestation periods (Trinkhaus 1984) and rapid in utero brain growth (Dean et al. 1986) in Neandertals have been rejected (see Stringer et al. 1990:148).
While pelvic inlet size during the Pleistocene appears to be, overall, similar to modern humans, cranial capacity increased. during the Middle and Late Pleistocene. Stasis in pelvic inlet size and increase in head size produces an "obstetric dilemma" where the fetal head is larger than the birth canal. Rosenberg and Trevathan (2002:1205) state that
"Two changes could have allowed an increase in adult brain size to occur: human infants could have been born with a smaller percentage of adult brain size (resulting in greater infant helplessness) and/or there could have been an alteration of the shape of the pelvis concomitant with a change in the mechanism of birth."
There is a third possibility: fetal head molding. The possible importance of fetal head molding in Neandertals is raised by similarities in both pelvic inlet size and adult cranial capacity to recent humans. Minugh-Purvis (1988:260) speculated that the thicker vault bone observed in Neandertal fetal remains would have posed a problem if delivery required a "considerable degree of head molding." The possibility was also discussed by Friedlander and Jorndan (1994).
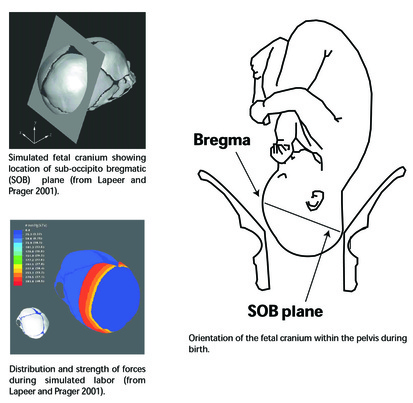 Figure 2.
Figure 2. In recent humans, the fetal cranium is a flexible structure that deforms during birth because of pressures between the fetal head and the cervical walls (Lapeer and Prager 2001; McPherson and Kriewall 1980a, 1980b) (Figure 2). Pressures and deformation are greatest at the sub-occipito bregmatic plane (Lapeer and Prager 2001; Rosenberg and Trevthan 2002).
During a normal labor, the parietal bones undergo the most significant changes in shape, being compressed towards each other and elongating in the axial plane (Lapeer and Prager 2001; McPherson and Kriewall 1980b). The occipital bone is relatively rigid and undergoes little change during molding (McPherson and Kriewall 1980b:18; Rosenberg and Trevathan 2002:1201). The frontal, occipital, and parietal bones interlock at the sutures after a certain limit of deformation occurs, preventing excessive molding and protecting the brain within a more rigid structure (McPherson and Kriewall 1980a:15).
The risk of excessive molding is greater in pre-term deliveries, where cranial bone is not sufficiently thick to prevent excessive molding (McPherson and Kriewall 1980a). Clinical studies have shown that excessive molding during birth (i.e., where too much deformation occurs) may be linked to psycho-neurological disorders, mental retardation, cerebral palsy, and death (see McPherson and Kriewall 1980b).
Fetal cranial bone must be thin enough to allow sufficient deformation of the cranium, but thick enough to form a rigid structure to protect the brain. Optimal thickness values would vary for different portions of the fetal cranium depending on the pressures that are exerted and the required responses to those pressures.
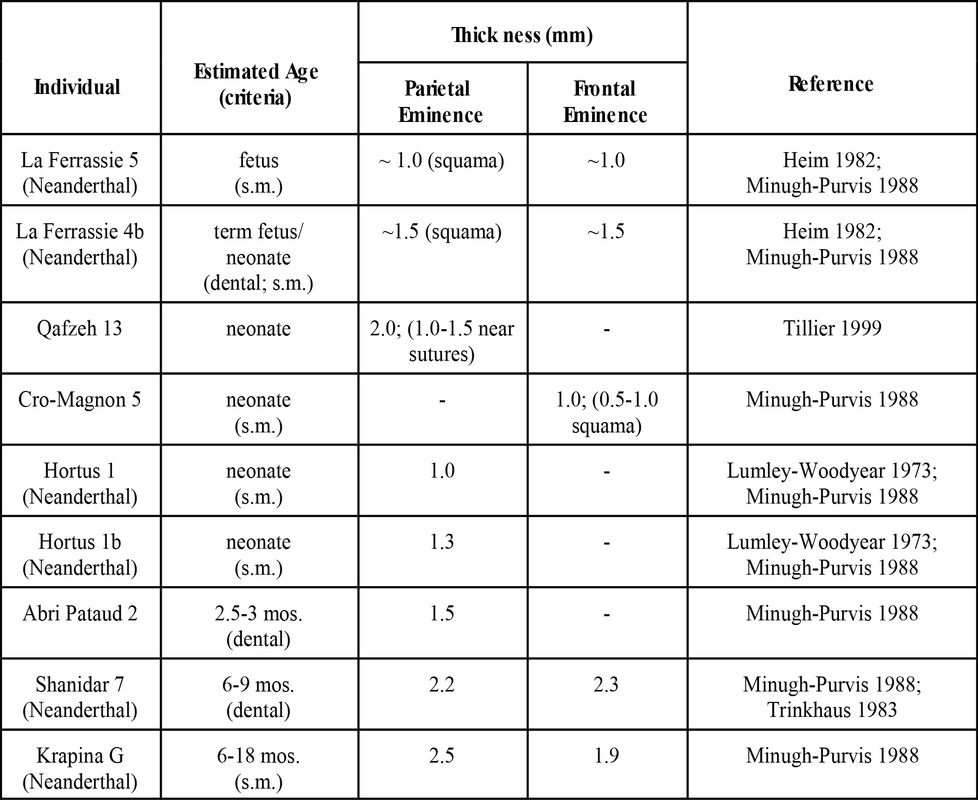
The Late Pleistocene fossil record contains numerous remains from sub-adult specimens. Of interest here are those remains that preserve portions of the cranial vault, particularly the frontal and parietal bones. Fetal and neonatal remains of Neandertals have been recovered from La Ferrassie (Heim 1982) and Hortus (Lumley-Woodyear 1973). Rremains of two Neandertals less than about a year old have been reported from Shanidar (Trinkhaus 1983) and Krapina (Minugh-Purvis 1988). Neonatal remains attributed to anatomically modern Homo sapiens have been reported from Cro-Magnon (Minugh-Purvis 1988), Qafzeh (Tillier 1999), and Abri Patuad (Minugh-Purvis 1988). Krapina is the earliest site, dating to the late Riss/early Wurm (Wolpoff 1999). La Ferrassie, Shanidar, Qafzeh, and Hortus date to Wurm I/Wurm II. Abri Pataud and Cro-Magnon date to Wurm III/IV (Wolpoff 1999).
Data on vault thickness at the parietal and frontal eminences are available for six fetal/neonate skeletons and three young (<1 year) infants from the Late Pleistocene.
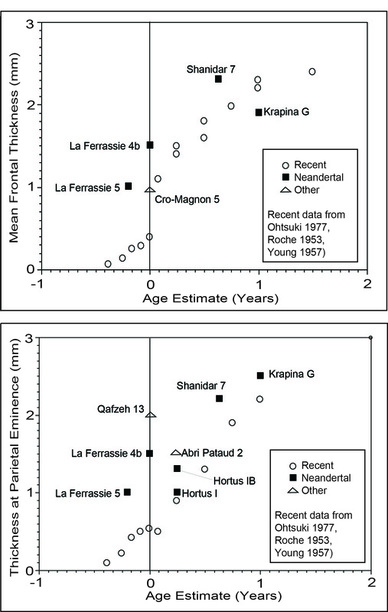 Figure 7.
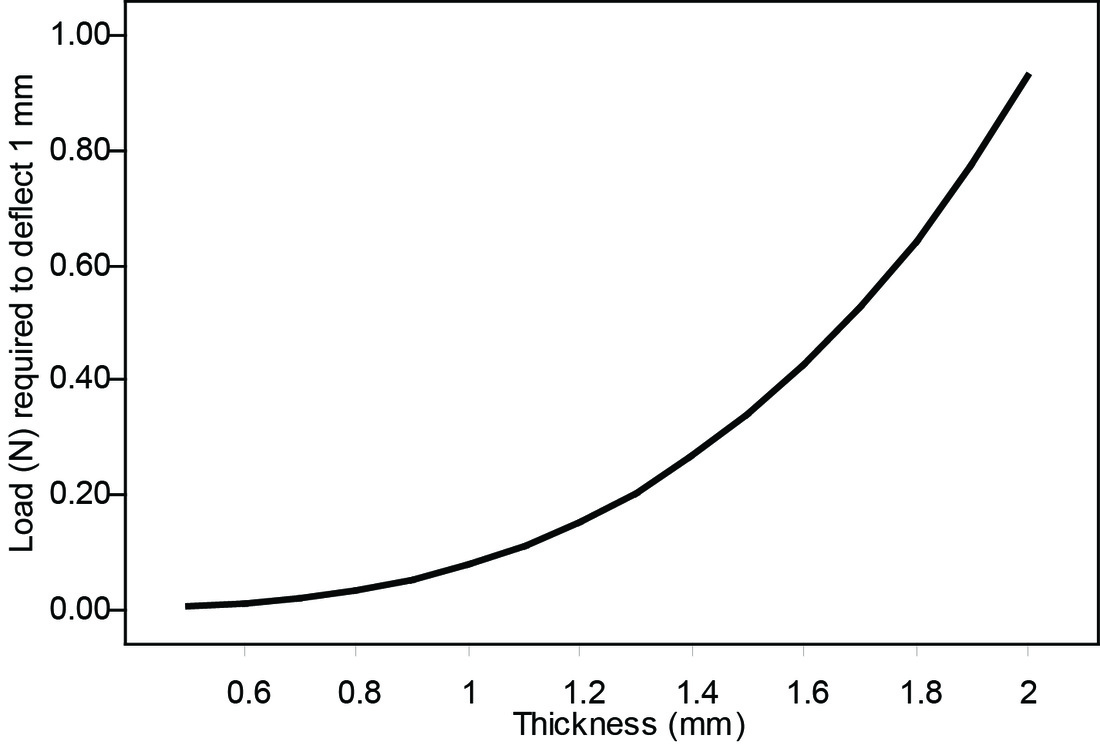
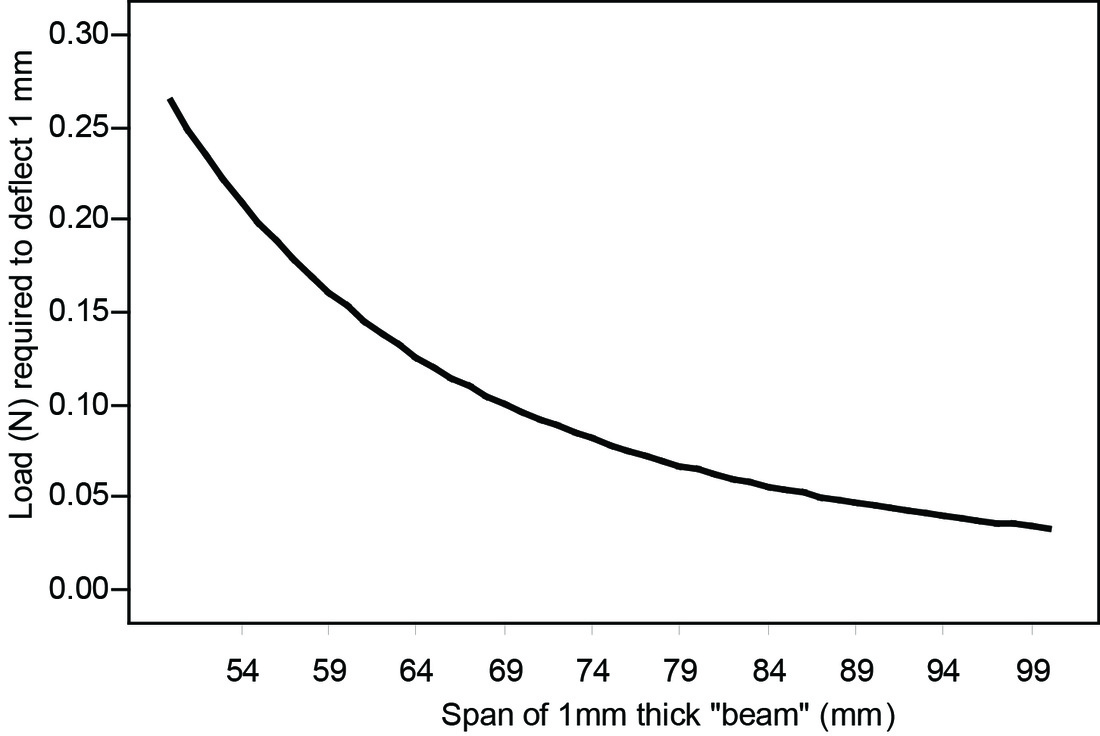
Figure 7. Considerably more force would be required to deform these bones, assuming the geometry of the bones was otherwise equivalent (see below).
Test prediction 1 is supported: differences in fetal cranial vault thickness are sufficient to affect molding of the cranium.
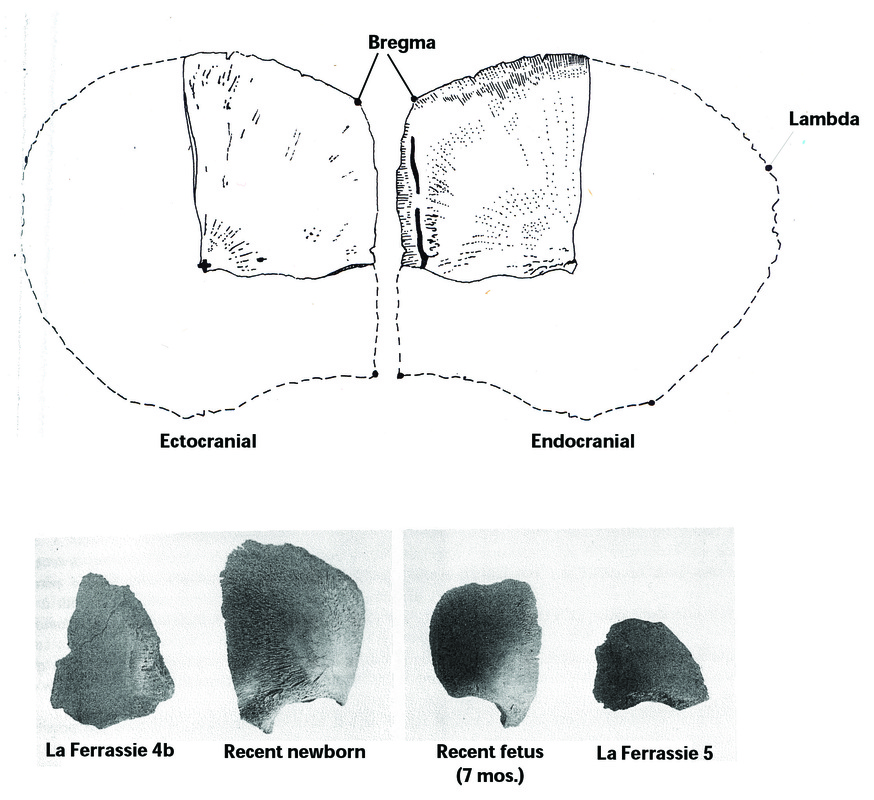
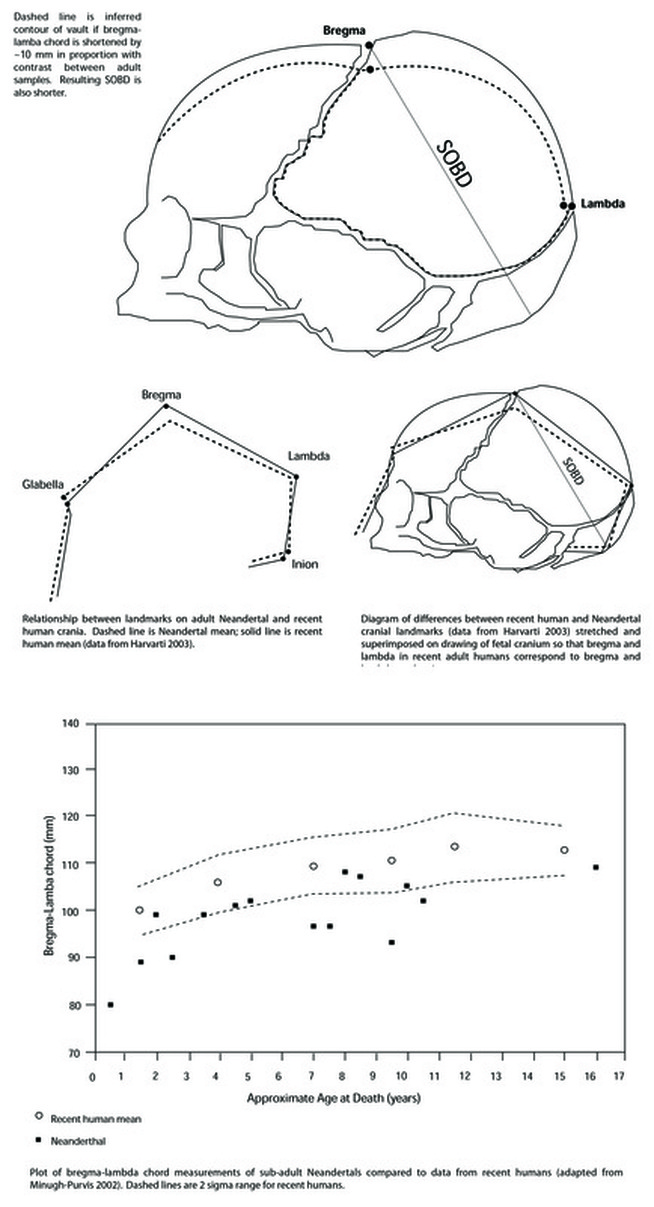
The limited data available on very young Late Pleistocene individuals suggests that some aspects of fetal head geometry may have differed from that of more recent humans (Minugh-Purvis 2002; Stringer et al. 1990). Bregma-lambda distance appears to have been shorter in Neandertals than in recent humans throughout life (see Minugh-Purvis 2002:488-489; Gunz and Havarti 2007; Harvarti 2003; Trinkhaus 1983:371). In mature Neandertals, the shorter distance is associated with a lower position of bregma (see Harvarti 2003)
Test prediction 2 is not supported: the dimensions of the fetal cranium are such that significant molding was probably not required for delivery.
Significant fetal head molding was probably not critical to successful Neanderthal birth. While thicker cranial bone would have reduced elasticity, a smaller SOBD would have negated or lessened the need for molding during birth.
Reduction in fetal cranial thickness may not have been a reproductive advantage for "modern" humans. Rather, cranial thinness associated with an increase in the SOBD may have increased the risks to the fetus during birth (i.e., though excessive molding) while reducing or maintaining the risk to both mother and fetus (i.e., through arrested labor). In the absence of selection for thinner bone associated with a flexibility requirement, thick fetal cranial bone would have offered protection to the fetal brain during delivery. Apparent stasis in pelvic anatomy suggests that smaller, thicker fetal crania may be the ancestral condition. An increase in SOBD, perhaps reflecting some difference in fetal brain growth, would have preceded selection for thinner cranial bone in this scenario.
The fetal cranium is a complex mechanical structure. Constructing a simulation model (similar to that of Lapeer and Prager 2001) of delivery in Neanderthals is possible with the available data. This model could be used to test hypotheses about obstetrics in a more sophisticated way than is possible by calculating simple ratios of head and pelvic size.
Dean, M.C., C. B. Stringer, and T. Bromage. 1986. Age at death of the Neanderthal child from Devil’s Tower, Gibraltar and the implications for studies of general growth and development in Neanderthals. American Journal of Physical Anthropology 70:301-309.
Friedlander, N. J., and D. K. Jordan. 1994. Obstetric implications of Neanderthal robusticity and bone density. Human Evolution 9:331-342.
Gunz, Philipp, and Katerina Havarti. 2007. The Neanderthal “chignon”: Variation, integration, and homology. Journal of Human Evolution 52:262-274.
Havarti, Katerina. 2003. The Neanderthal taxonomic position: Models of intra- and inter-specific craniofacial variation. Journal of Human Evolution 44:107-132.
Heim, Jean-Louis. 1982. Les enfants nJandertaliens de La Ferrassie. Paris, Masson.
Lapeer, R.J., and R.W. Prager. 2001. Fetal head moulding: Finite element analysis of a fetal skull subjected to uterine pressures during the first stage of labour. Journal of Biomechanics 34:1125-1133.
Lumley-Woodyear, Marie-Antionette de. 1973. AntenJanderthaliens et NJandertaliens du bassin Mediterraneen occidental europen. Etudes Quaternaires. MJmoire 2, Marseille, UniversitJ de Provence.
McPherson, Gregg K., and Timothy J. Kriewall. 1980a. The elastic modulus of fetal cranial bone: A first step towards an understanding of the biomechanics of fetal head molding. Journal of Biomechanics 13:9-16.
McPherson, Gregg K., and Timothy J. Kriewall. 1980b. Fetal head molding: An investigation utilizing a finite element model of the fetal parietal bone. Journal of Biomechanics 13(1):17-26.
Minugh-Purvis, Nancy. 1988. Patterns of craniofacial growth and development in Upper Pleistocene hominids. PhD dissertation, University of Pennsylvania.
Minugh-Purvis, Nancy. 2002. Heterochronic change in the neurocranium and the emergence of modern humans. In Human evolution through developmental change, edited by Nancy Minugh-Purvis and Kenneth J. McNamara, pp. 479-498. Baltimore: Johns Hopkins University Press.
Nelson, Andrew J., and Jennifer L. Thompson. 2002. Adolescent postcranial growth in Homo neanderthalensis. In Human evolution through developmental change, edited by Nancy Minugh-Purvis and Kenneth J. McNamara, pp. 442-463. Baltimore: Johns Hopkins University Press.
Ohtsuki, Fumio. 1977. Developmental changes of the cranial bone thickness in the human fetal period. American Journal of Physical Anthropology 46:141-154.
Rak, Y., and B. Arensburg. 1987. Kebara 2 Neandertal pelvis: First look at a complete inlet. American Journal of Physical Anthropology 73:227-231.
Roche, A. F. 1953. Increase in cranial thickness during growth. Human Biology 25(2):81-92.
Rosenberg, Karen R. 1992. The evolution of modern human childbirth. Yearbook of Physical Anthropology 35:89-124
Rosenberg, Karen R. 1998. Morphological variation in west Asian postcrania. In Neandertals and modern humans in western Asia, edited by Takeru Akazawa, Kenichi Aoki, and Ofer Bar-Yosef, pp. 367-379. Plenum, New York.
Rosenberg, Karen, and Wenda Trevathan. 2002. Birth, obstetrics and human evolution. BJOG: an International Journal of Obstetrics and Gynaecology 109:1199-1206.
Ruff, Christopher B. 1995. Biomechanics of the hip and birth in early Homo. American Journal of Physical Anthropology 98:527-574.
Stringer, Christopher B., M. Chistopher Dean, and Robert D. Martin. 1990. A comparative study of cranial and dental development within a recent British samples and among Neandertals. In Primate life history and evolution, edited by C. Jean DeRousseau, pp. 115-152. New York: Wiley-Liss.
Tillier, Anne-Marie. 1998. Onotogenetic variation in Late Pleistocene Homo sapiens from the Near East. In Neandertals and modern humans in western Asia, edited by Takeru Akazawa, Kenichi Aoki, and Ofer Bar-Yosef, pp. 381-389. Plenum, New York.
Tillier, Anne-Marie. 1999. Les enfants mousteriens de Qafzeh: Interpretation phylogenetique et paleoauxologique. Cahiers de Paleoanthropologie. Paris, CNRS Editions.
Trinkhaus, E. 1983. The Shanidar Neanderthals. New York: Academic Press.
Trinkhaus, E. 1984 Neandertal pubic morpohology and gestation length. Current Anthropology 25:509-514.
Trinkhaus, Erik, and Robert L. Tompkins. 1990. The Neandertal life cycle: The possibility, probability, and perceptibility of contrasts with recent humans. In Primate Life History and Evolution, edited by C. Jean DeRousseau, pp. 153-180. New York: Wiley-Liss.
Young, Richard W. 1957. Postnatal growth of the frontal and parietal bones in white males. American Journal of Physical Anthropology 15:367-386.
Wolpoff, Milford H. 1999. Paleoanthropology. 2nd edition. Boston: McGraw-Hill.


 RSS Feed
RSS Feed
